At Climb, we empower patients, consumers, and the members of their care teams, to make better decisions about their health by accelerating access to products that have been tested and proven effective through clinical research. Our platform enables us to serve this objective by expanding access to high-quality clinical research to more companies and by expanding capacity within the clinical industry to reliably execute study protocols.
More specifically, our platform allows for custom clinical trial protocols to be configured and managed at scale through the use of modular components and customizable study templates. Once configured, we are able to automate key pieces of the project plan, which, in turn, minimizes or eliminates the manual work required of study teams and allows the participant to drive their own forward progress in the study. When the study team’s attention is required, task-oriented dashboards populated by the Climb system bring clarity to their workdays.
Climb tracks trial progress in real-time and reports are always available to everyone involved. That means study teams spend less time updating stakeholders and more time executing. Together, these product capabilities allow us to redefine the clinical trial process, from planning through data analysis, unlocking new research opportunities and improved outcomes for everyone involved.
Configuring your Workflow
All clinical trials are, by definition, unique and different from other trials having come before them, but that should not mean every trial needs to be built from scratch. With Climb, custom project workflows can be quickly designed using modular components, allowing for complex event schedules to be created in minutes. Organize the data collection modules, such as appointments and assessments, across a project timeline and layer in the automated communications and tasks to ensure the work gets done on time every time.
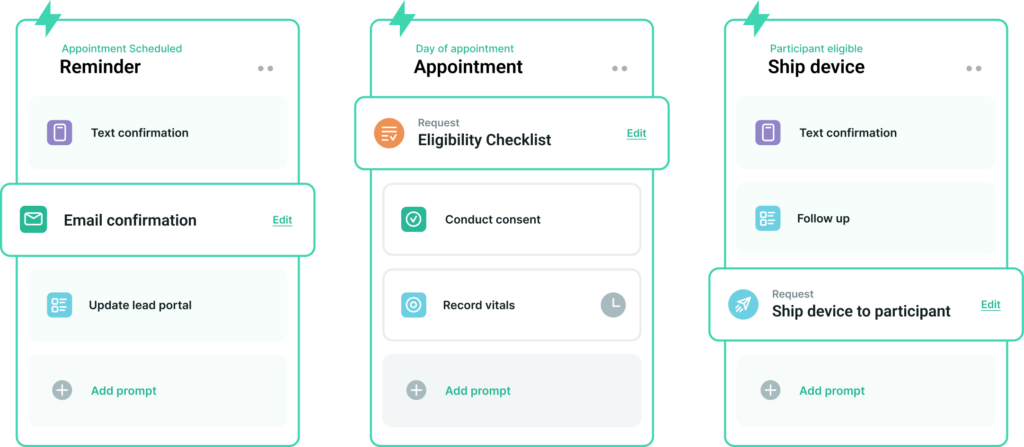
Climb’s software can revamp one-off burdensome operations conducted at research sites just as easily as it can be configured to execute trial protocols for remote, hybrid, or site-based clinical trials. Our customers enjoy flexible workflow designs tailored to meet the unique needs of their projects without lengthy start-up times.
Automating Study Activities
Configuration and workflow execution take place in the same platform to accelerate progress while preserving the study team’s visibility and control. Automated scoring can be used to pre-screen candidates entering a recruitment pipeline or to measure outcomes reported through in-trial assessments, and next steps queue instantly.
Communications, often a key driver of study adherence, are automated at key milestones to notify participants of outstanding action items or of upcoming next steps. All without the study team lifting a finger.
Workflow automation allows for standardized participant experiences and reduced operational workloads managed by study teams. The team can trust the system to handle the tedious and repetitive portions of their day, giving them time to focus on the parts of the process that need them most.
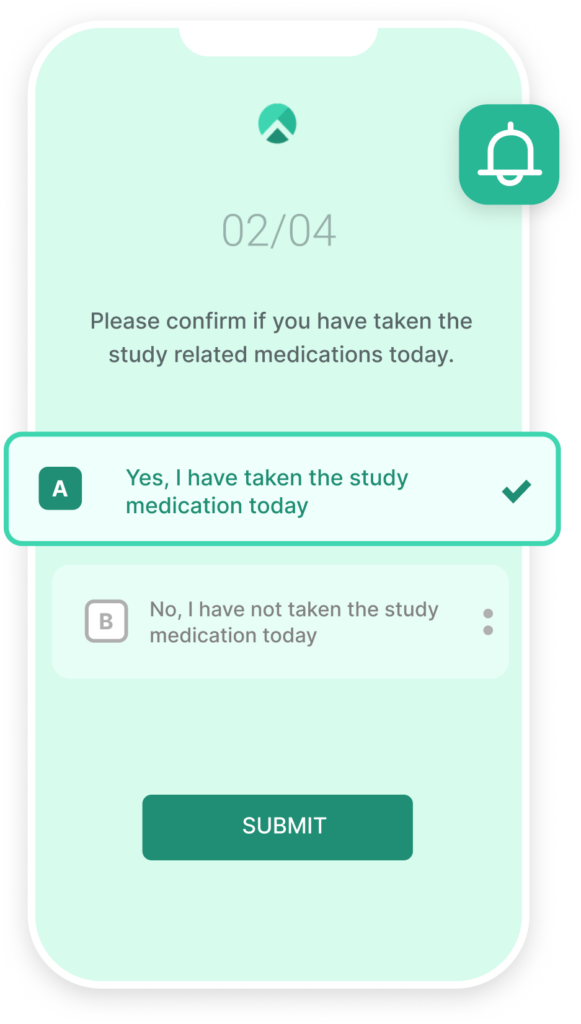
Organizing Study Team Tasks
Some study tasks simply require a human touch. Climb provides study teams with a centralized, organized, and actionable task list to streamline the countless unique trial operations that must be managed throughout a given protocol. These task-oriented dashboards bring clarity to the workday of study team members by conveying the prioritized set of responsibilities of each individual or team.
When participants or study teams fail to complete milestones, tasks automatically escalate to the most appropriate contact. If, for example, the participant or coordinator reports a change in non-study medications, review of new medications will be directly assigned as a task to the overseeing physician or Principal Investigator for approval or study discontinuance. By assigning and organizing the discrete set of trial activities relevant to each study team member within a single platform, project teams maximize productivity. Additionally, trials may be conducted by distributed teams while maintaining high reliability and accuracy standards.
Monitoring Study Progress
Understanding the health and progress of the study is vital to stakeholders as they seek to make informed decisions about project timelines and budgets. The Climb dashboard provides a holistic perspective of the project’s status with valuable drill-down capabilities. Full-funnel reports track the total number of engaged candidates throughout every possible outcome across the entire enrollment and treatment journey. It becomes easy to see if participants are propagating through the system as expected or if they are piling up or failing at higher than anticipated rates at a specific study milestone. This helps the study team quickly identify issues such as low qualification rates, high dropout rates, delinquent or disengaged participants, and overall completion rates.
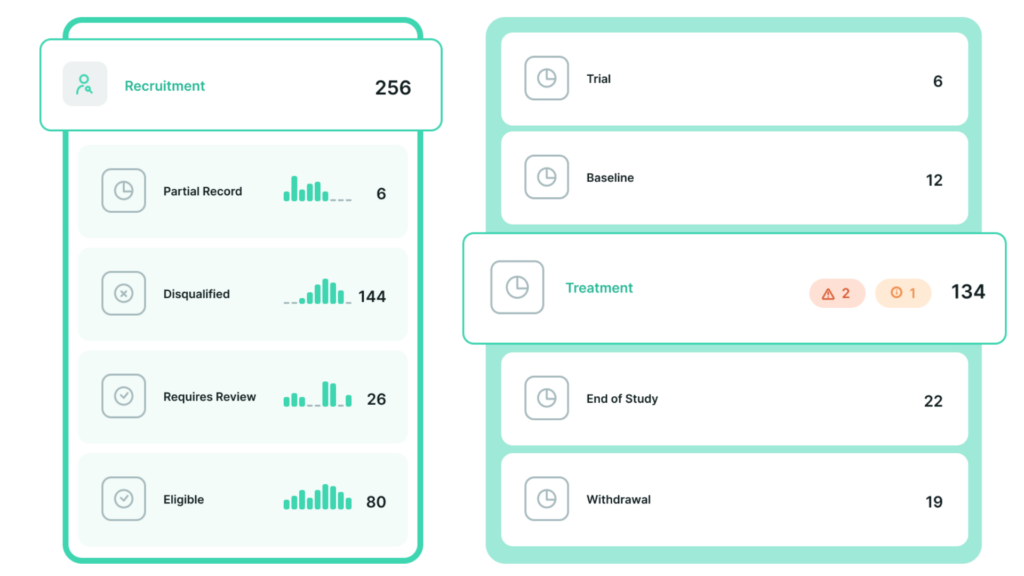
At the site level, these insights may impact marketing and workflow optimizations. When reviewed by the sponsor or CRO, they provide key insights impacting potential protocol amendments and study team adjustments.
Key Results
With Climb’s scalable software solution, research teams large and small gain access to the high-quality clinical data needed to prove the efficacy of their products on a timeline and budget they can afford. However, the democratization of research does not just serve those conducting it. Most importantly, it serves every one of us seeking to make the best decisions possible about our own health and wellness.
To see for yourself how the Climb platform works and to learn more about our process, schedule a demo today!
