Sumayah Nuhaily
If anyone were asked about their first experience in managing a clinical research trial, they would almost certainly say that it was a complex process. Even those sponsors with previous experience face new challenges with each subsequent trial they manage because of the inherent uniqueness of each research protocol. The Food and Drug Administration (FDA), which is the governing body in charge of approving drugs and devices in the United States, outlines very specific requirements that an industry Sponsor must meet before their drug or device may be marketed and sold. Their website contains dozens of guidebooks and federal codes related to how clinical trials should be carried out, all of which are far from reader-friendly.
As a result, Sponsors are often compelled to outsource their clinical trials to companies that have years or even decades of experience in conducting clinical trials, with the hope of gathering data as evidence to submit to FDA for product approval. The outsourcing process can be complicated in itself, as Sponsors need to find the outsourcing model and company that would work best for them depending on their exact budget and trial needs.
With three outsourcing models, including full-service outsourcing, outsourcing to functional service providers, and hybrid outsourcing models, and countless varieties of potential partner organizations, it can be challenging to know where, and how, to get started. The purpose of our guide to outsourcing clinical trials is to assist Sponsors in making those initial tough decisions.
Overview of Outsourcing in Clinical Trials
With its origins tracing back to the 1970s, clinical trial outsourcing is not a new concept by any means; however, the recent COVID-19 pandemic contributed to a heightened awareness of the strategy. Vaccines, antivirals, and antibodies needed to be developed and approved as rapidly as possible in the interest of public health, and the traditional model of pharmaceutical and biotechnology in-house testing would have taken years to bring the life-saving drugs to the people who needed them.
The pharma and biotech companies needed to reach a vast number of trial sites and human participants in order to carry out the research on such a large scale, and the practice of outsourcing and creating a network of trial sites became critical to the success of the therapies. Industry Sponsors continue to realize the high value of outsourcing their clinical trial workloads: the global market for contract research outsourcing is expected to continue growing rapidly; with a value of $43bn in 2023, it is likely to reach $65bn by 2030 (Source: Vision Research Reports).
Clinical trial outsourcing is not one-size-fits-all, however, which is why more than one model exists. In the next section, we will describe each model and outline the main differences between them.
Clinical Trial Outsourcing Models
Full-Service Outsourcing (FSO)
| AKA | End-to-end |
| Methodology | Planning and execution managed by provider |
| System(s) | Predominantly provider |
| Accountability | Predominantly provider (Sponsor influence varies) |
This model provides outsourcing of the clinical trial’s components on a project and/or program basis. Sponsors are not involved in the planning and execution of the trial, and only become involved in ethical compliance matters and communication with FDA on an as-needed basis. Full-service is typically selected by Sponsors that do not have previous clinical research experience or prefer to focus solely on the in-house development of their products.
Functional Service Providers (FSPs)
| AKA | Functional service provision |
| Methodology | Strategic capacity management |
| System(s) | Sponsor |
| Accountability | Sponsor |
The FSP model allows Sponsors to choose specific trial activities they would like to outsource (think a-la-carte) and to whom they would like to contract them out. An important distinction between FSO and FSP is that the former model places the majority of the control in the hands of the provider, while the latter embeds the outsourced services into the Sponsors’ existing infrastructure.
This is generally helpful if the Sponsor needs coverage for a highly specialized task (e.g., data analysis, regulatory management, or medical writing) and does not want to be restricted to a single provider or feel the need to outsource the entirety of the trial.
Hybrid Model
| AKA | Mixed; blended |
| Methodology | Functional capacity management |
| System(s) | Both Sponsor and provider |
| Accountability | Both Sponsor and provider |
The hybrid model of outsourcing is a blend of FSO and FSP and is customizable depending on the Sponsor’s preference and/or needs. It provides the highest degree of flexibility and does not restrict the Sponsor in seeking exactly what they need and from whom they need it. This is an increasingly common model for Sponsor companies to adopt, as it is typically the most cost-effective of the three.
Pros and Cons of Outsourcing Clinical Trials
Benefits
The first, and likely most appealing, benefit to outsourcing clinical trials is that it is far less operationally burdensome for Sponsors in running and completing their research. It allows them to focus more on the in-house development of their products and worry less about the staff, facilities, and time required to carry out a trial on their own.
Outsourcing also helps fill geographic gaps that might otherwise create a very difficult situation with the recruitment of study participants. For example, if a drug is developed to treat a rare disease that occurs in 1% of the population, it would be impossible to recruit 300 participants within a 100 mile radius of the Sponsor’s physical location. Transporting nationwide candidates to the Sponsor site for the duration of the trial would be cost-prohibitive and time-intensive, but contracting with trial sites located across the country would guarantee access to hundreds of thousands of potential trial participants.
Another clear benefit to outsourcing is the opportunity to leverage operational and therapeutic expertise. Some Sponsors work with hospitals for this reason; they are able to secure access to doctors that specialize in the therapeutic indication that their drug or device aims to treat, and additionally gain access to facilities that are set up to screen and monitor that indication. Electronic medical record (EMR) and clinical trial management system (CTMS) software are costly and require extensive experience to manage, so contracting with clinical facilities effectively erases the burden they would otherwise create for the Sponsor.
Sponsors’ experience with data analysis and medical writing is often limited if they have not carried out clinical research on their own, so they often enlist the help of other providers, including contract research organizations (CROs), that have entire departments dedicated to each task. This helps ensure that the trial has a high probability of succeeding and bringing high-quality data and reporting to FDA when approval is sought by the Sponsor.
Possible Challenges
Although the above benefits may make outsourcing clinical trials seem idyllic, that is sometimes far from the truth. It is in a Sponsor’s best interest to tread cautiously when choosing partners to manage their research, as they are ultimately responsible for everything that develops in the trial, and the end result contributes directly to the success of their company. Developing trust is an essential precursor to contracting with providers.
While the FSO model is convenient because it removes the Sponsor’s operational burden, it can be costly for the same reason. Hiring an organization to carry out every study component is often prohibitively expensive for smaller and mid-size Sponsors, which is why the model is typically adopted by more established and very large pharma and biotech companies.
When using the FSP or hybrid models of outsourcing, it becomes critical to balance the management responsibilities amongst each provider and the Sponsor itself. It is important that clear roles for each trial component are laid out before the trial process begins so that there are no gaps or overlap in responsibilities during its execution.
More specific to hybrid models is the challenge of balancing the in-house and outsourced study tasks, with the ideal balance allowing Sponsors to comfortably and promptly carry out their portion of the trial while maintaining their usual in-house product development schedule. The success of the hybrid model is often determined by this balance.
Making the Decision to Outsource
With many moving parts and the stakes so high, deciding on a model to outsource is not an easy decision. Moving forward with the outsourcing model that is best for a Sponsor will depend on a few factors, all of which are fairly straightforward to assess.
Research Resources
The resources required to carry out a clinical trial are undoubtedly extensive. Before outsourcing trial tasks, it is best to have a complete picture of what the Sponsor has to offer internally. Some Sponsors will have few resources to contribute to the research, while others may be able to reasonably take on a few components.
Important resources to consider are participant recruitment methods, facilities and equipment, coordinators and managers, medical professionals, regulatory bodies, statisticians, and medical writers.
Past Experience
The value of research expertise when managing a clinical trial cannot be overstated, because, as we mentioned in the opening of the guide, FDA requirements for clinical trials are numerous, complex, and leave no room for error. It is especially important to plan the trial with experienced researchers, as trial methods and statistical plans must be well-engineered to set up the trial for success.
Budget
This is, of course, an important consideration to each and every industry Sponsor. Clinical trials vary greatly in cost and depend primarily on clinical procedures, staffing expenses, and site monitoring services.
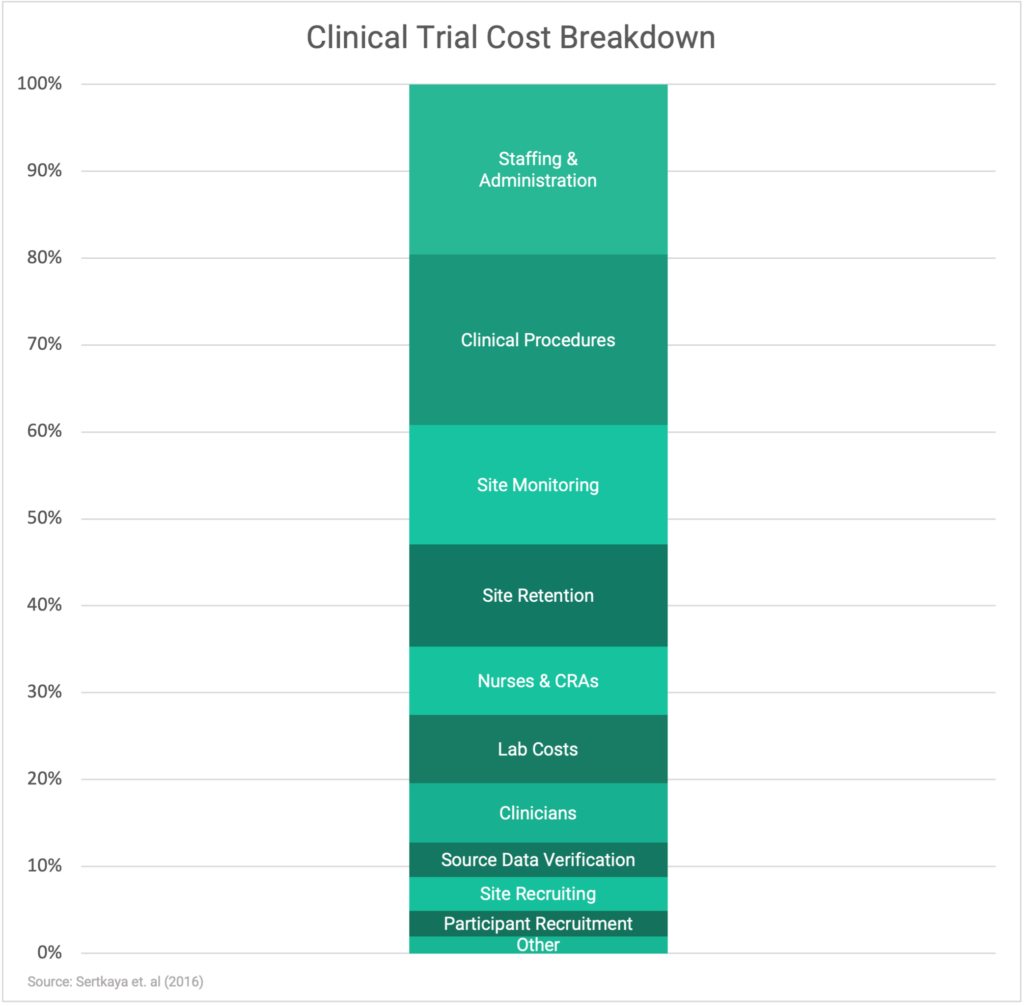
While adopting the FSO model of outsourcing may be difficult for some to afford, the FSP and hybrid models allow Sponsors to choose specific services that would fill their in-house processes’ gaps while being mindful of expenditures. Some Sponsors elect to hire temporary teams internally during clinical trials, which certainly requires more effort and expertise but could help control the costs when compared to outsourcing administrative services to a contracted research provider.
Beginning the Process of Outsourcing
Clinical trials can be difficult to navigate, and making the choice to outsource clinical trial activities can help Sponsors dedicate the majority of their in-house resources to the development of their products.
We built Climb, a hybrid outsourcing service, to make clinical research feasible and simple for everyone. The Climb platform helps to automate the operational complexities associated with clinical trial research, creating a seamless experience for trial participants, study team members, and trial Sponsors from start to finish. Additionally, our research team and network of third-party providers are able to support countless individual clinical operations needs. Climb’s direct-to-participant platform alongside the integrated partner network makes low-overhead research available to even the busiest and most cost conscious teams.
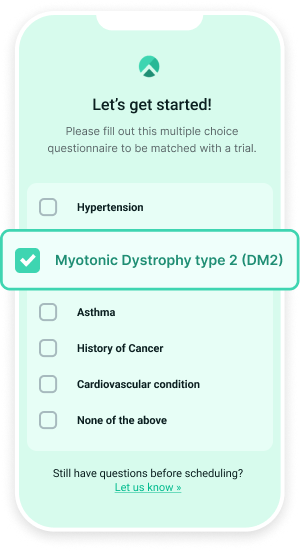
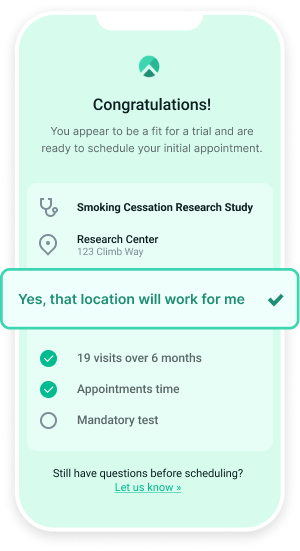

To learn more about how Climb may be able to address your clinical trial needs, schedule a demo today.
